CONTACT US
CONTACT US
CUSTOMER SERVICE
For ordering, billing, or finding your local
sales representative,
call 1.800.367.5737 or email
PRMOrder@allergan.com
ONLINE ORDERING
Click here: Allergan DirectCLINICAL AND MEDICAL ASSISTANCE
Call 1.800.678.1605 or visit our
Medical Information website
REIMBURSEMENT SUPPORT
Call 1.888.543.3656 or email
AllerganPRM@thepinnaclehealthgroup.com
AlloDermTM Regenerative Tissue Matrix
portfolio of products
AlloDermTM RTM is an acellular dermal matrix (ADM) that has been trusted with more than 3 million implantations.1 No other ADM has more publications, with hundreds of scientific* and clinical articles.2 And no other ADM has the extensive experience of AlloDermTM RTM—25 years and counting.3 AlloDermTM RTM offers a wide range of products to meet your needs



Sterile4



Zero documented disease transmissions5



Ready to use with a minimum 2-minute soak4



Able to be stored without refrigeration4



Perforated

The AlloDermTM Product Selection Assistant makes it easy to select the product you need. Choose the shape, size, texture, and thickness, and the Assistant will identify the specific product code. Just follow the steps on-screen to select your products and create a customized product list.
Get Started
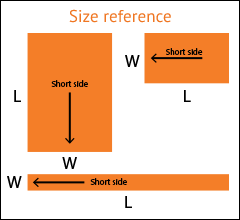
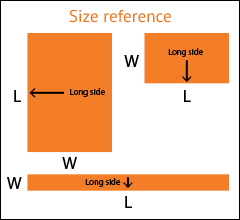


Select the arrow to drop-down a full list of available widths.
If your exact desired dimension is not shown, select the closest one.
Select the arrow to drop-down a full list of available lengths.
Don't see your size? Call 1.800.367.5737 for other available sizes.
Perforated Contour: Sizes and Thicknesses
AlloDerm SELECT™
Regenerative Tissue
Matrix
![]() Perforated Contour
Perforated Contour
| Product description | Piece size (cm) | Coverage (cm2) | Thickness | Thickness range (mm) | Product code |
|---|---|---|---|---|---|
| AlloDerm SELECT™ Regenerative Tissue
Matrix
|
X-Large 11.8 x 23.7 |
200 | Medium | 1.6 ± 0.4 | CXL1518P |
| Thick | 2.4 ± 0.4 | CXL1519P | |||
| Large 10.7 x 21.5 |
164 | Thin | 1.0 ± 0.2 | CL1516P | |
| Medium | 1.6 ± 0.4 | CL1518P | |||
| Thick | 2.4 ± 0.4 | CL1519P | |||
| Medium 9.6 x 19.3 |
132 | Thin | 1.0 ± 0.2 | CM1516P | |
| Medium | 1.6 ± 0.4 | CM1518P | |||
| Thick | 2.4 ± 0.4 | CM1519P | |||
| Small 7.3 x 14.7 |
77 | Thin | 1.0 ± 0.2 | CS1516P | |
| Medium | 1.6 ± 0.4 | CS1518P | |||
| Thick | 2.4 ± 0.4 | CS1519P |
Contour: Sizes and Thicknesses
AlloDerm SELECT™
Regenerative Tissue
Matrix
![]() Contour
Contour
| Product description | Piece size (cm) | Coverage (cm2) | Thickness | Thickness range (mm) | Product code |
|---|---|---|---|---|---|
| AlloDerm SELECT™ Regenerative Tissue
Matrix
|
X-Large 11.8 x 23.7 |
200 | Medium | 1.6 ± 0.4 | CXL1518 |
| Thick | 2.4 ± 0.4 | CXL1519 | |||
| Large 10.7 x 21.5 |
164 | Thin | 1.0 ± 0.2 | CL1516 | |
| Medium | 1.6 ± 0.4 | CL1518 | |||
| Thick | 2.4 ± 0.4 | CL1519 | |||
| Medium 9.6 x 19.3 |
132 | Thin | 1.0 ± 0.2 | CM1516 | |
| Medium | 1.6 ± 0.4 | CM1518 | |||
| Thick | 2.4 ± 0.4 | CM1519 | |||
| Small 7.3 x 14.7 |
77 | Thin | 1.0 ± 0.2 | CS1516 | |
| Medium | 1.6 ± 0.4 | CS1518 | |||
| Thick | 2.4 ± 0.4 | CS1519 |
RESTORE Perforated: Sizes and Thicknesses
AlloDerm SELECT™
Regenerative Tissue
Matrix
 RESTORE Perforated
RESTORE Perforated
| Product description | Piece size (cm) | Coverage (cm2) | Thickness | Thickness range (mm) | Product code |
|---|---|---|---|---|---|
AlloDerm SELECT RESTORE™ Regenerative Tissue Matrix
 RESTORE Perforated
RESTORE Perforated
|
Large 20.1 x 23.6 |
327 | Medium | 1.6 ± 0.4 | RL1518P |
| Thick | 2.4 ± 0.4 | RL1519P | |||
| X-Thick | 3.4 ± 0.6 | RL1522P |
RESTORE: Sizes and Thicknesses
AlloDerm SELECT™
Regenerative Tissue
Matrix
![]() RESTORE
RESTORE
| Product description | Piece size (cm) | Coverage (cm2) | Thickness | Thickness range (mm) | Product code |
|---|---|---|---|---|---|
| AlloDerm SELECT RESTORE™ Regenerative Tissue Matrix
|
Large 20.1 x 23.6 |
327 | Medium | 1.6 ± 0.4 | RL1518P |
| Thick | 2.4 ± 0.4 | RL1519P | |||
| X-Thick | 3.4 ± 0.6 | RL1522P |
Perforated Rectangle: Sizes and Thicknesses
AlloDerm SELECT™
Regenerative Tissue
Matrix
![]() Perforated Rectangle
Perforated Rectangle
| Product description | Piece size (cm) | Coverage (cm2) | Thickness | Thickness range (mm) | Product code |
|---|---|---|---|---|---|
| AlloDerm SELECT™ Regenerative Tissue
Matrix
|
16 x 20 | 320 | Medium | 1.6 ± 0.4 | 1518320P |
| Thick | 2.4 ± 0.4 | 1519320P | |||
| X-Thick | 3.4 ± 0.6 | 1522320P |
Rectangle: Sizes and Thicknesses
AlloDerm SELECT™
Regenerative Tissue
Matrix
![]() Rectangle
Rectangle
| Product description | Piece size (cm) | Coverage (cm2) | Thickness | Thickness range (mm) | Product code |
|---|---|---|---|---|---|
| AlloDerm SELECT™ Regenerative Tissue
Matrix
|
16 x 20 | 320 | Medium | 1.6 ± 0.4 | 1518320 |
| Thick | 2.4 ± 0.4 | 1519320 | |||
| X-Thick | 3.4 ± 0.6 | 1522320 | |||
| 12 x 20 | 240 | Medium | 1.6 ± 0.4 | 15181220 | |
| Thick | 2.4 ± 0.4 | 15191220 | |||
| 12 x 16 | 192 | Medium | 1.6 ± 0.4 | 15181216 | |
| Thick | 2.4 ± 0.4 | 15191216 | |||
| 12 x 12 | 144 | Medium | 1.6 ± 0.4 | 1418144 | |
| Thick | 2.4 ± 0.4 | 1419144 | |||
| X-Thick | 3.4 ± 0.6 | 1422144 | |||
| 10 x 20 | 200 | Medium | 1.6 ± 0.4 | 15181020 | |
| Thick | 2.4 ± 0.4 | 15191020 | |||
| 10 x 16 | 160 | Medium | 1.6 ± 0.4 | 15181016 | |
| Thick | 2.4 ± 0.4 | 15191016 | |||
| 8 x 20 | 160 | Medium | 1.6 ± 0.4 | 1518160 | |
| Thick | 2.4 ± 0.4 | 1519160 | |||
| X-Thick | 3.4 ± 0.6 | 1522160 | |||
| 8 x 16 | 128 | Thin | 1.0 ± 0.2 | 1516128 | |
| Medium | 1.6 ± 0.4 | 1518128 | |||
| Thick | 2.4 ± 0.4 | 1519128 | |||
| X-Thick | 3.4 ± 0.6 | 1522128 | |||
| 6 x 18 | 108 | Medium | 1.6 ± 0.4 | 1518108 | |
| Thick | 2.4 ± 0.4 | 1519108 | |||
| X-Thick | 3.4 ± 0.6 | 1522108 | |||
| 6 x 16 | 96 | Thin | 1.0 ± 0.2 | 1516616 | |
| Medium | 1.6 ± 0.4 | 1518616 | |||
| Thick | 2.4 ± 0.4 | 1519616 | |||
| X-Thick | 3.4 ± 0.6 | 1522616 | |||
| 8 x 12 | 96 | Medium | 1.6 ± 0.4 | 151896 | |
| Thick | 1.6 ± 0.4 | 151996 | |||
| X-Thick | 3.4 ± 0.6 | 152296 | |||
| 6 x 12 | 72 | Thin | 1.0 ± 0.2 | 151672 | |
| Medium | 1.6 ± 0.4 | 151872 | |||
| Thick | 2.4 ± 0.4 | 151972 | |||
| X-Thick | 3.4 ± 0.6 | 152272 | |||
| 4 x 16 | 64 | Thin | 1.0 ± 0.2 | 151664 | |
| Medium | 1.6 ± 0.4 | 151864 | |||
| Thick | 2.4 ± 0.4 | 151964 | |||
| X-Thick | 3.4 ± 0.6 | 152264 | |||
| 5 x 10 | 50 | Thin | 1.0 ± 0.2 | 141650 | |
| Medium | 1.6 ± 0.4 | 141850 | |||
| Thick | 2.4 ± 0.4 | 141950 | |||
| X-Thick | 3.4 ± 0.6 | 142250 | |||
| 4 x 12 | 48 | X-Thin | 0.55 ± 0.25 | 141448 | |
| Thin | 1.0 ± 0.2 | 151648 | |||
| Medium | 1.6 ± 0.4 | 151848 | |||
| Thick | 2.4 ± 0.4 | 151948 | |||
| X-Thick | 3.4 ± 0.6 | 152248 | |||
| 4 x 7 | 28 | X-Thin | 0.55 ± 0.25 | 141428 | |
| Thin | 1.0 ± 0.2 | 141628 | |||
| Medium | 1.6 ± 0.4 | 141828 | |||
| Thick | 2.4 ± 0.4 | 141928 | |||
| X-Thick | 3.4 ± 0.6 | 142228 | |||
| 2 x 12 | 24 | X-Thick | 3.4 ± 0.6 | 142224 | |
| 3 x 7 | 21 | Thin | 1.0 ± 0.2 | 141621 | |
| Medium | 1.6 ± 0.4 | 141821 | |||
| Thick | 2.4 ± 0.4 | 141921 | |||
| X-Thick | 3.4 ± 0.6 | 142221 | |||
| 2 x 4 | 8 | X-Thin | 0.55 ± 0.25 | 141408 | |
| Thin | 1.0 ± 0.2 | 141608 | |||
| Medium | 1.6 ± 0.4 | 141808 | |||
| Thick | 2.4 ± 0.4 | 141908 | |||
| X-Thick | 3.4 ± 0.6 | 142208 | |||
| 1 x 4 | 4 | X-Thin | 0.55 ± 0.25 | 141404 | |
| Thin | 1.0 ± 0.2 | 141604 | |||
| Medium | 1.6 ± 0.4 | 141804 | |||
| Thick | 2.4 ± 0.4 | 141904 | |||
| X-Thick | 3.4 ± 0.6 | 142204 | |||
| 1 x 2 | 2 | X-Thin | 0.55 ± 0.25 | 141402 | |
| Thin | 1.0 ± 0.2 | 141602 | |||
| Medium | 1.6 ± 0.4 | 141802 | |||
| Thick | 2.4 ± 0.4 | 141902 | |||
| X-Thick | 3.4 ± 0.6 | 142202 | |||
| 1 x 1 | 1 | X-Thick | 3.4 ± 0.6 | 142201 |
Graftable Rectangle: Sizes and Thicknesses
AlloDerm SELECT™
Regenerative Tissue
Matrix Fenestrated Rectangle
Fenestrated Rectangle
| Product description | Piece size (cm) | Coverage (cm2) | Thickness | Thickness range (mm) | Product code |
|---|---|---|---|---|---|
AlloDerm SELECT™ Regenerative Tissue
Matrix
 Fenestrated Rectangle Fenestrated Rectangle
|
4 x 4 | 16 | Thin | 1.0 ± 0.2 | 141616F |
| 4 x 8 | 32 | Thin | 1.0 ± 0.2 | 141632F | |
AlloDerm SELECT™
Regenerative Tissue
Matrix
 Meshed Rectangle
Meshed Rectangle
| Product description | Piece size (cm) | Coverage (cm2) | Thickness | Thickness range (mm) | Product code |
|---|---|---|---|---|---|
AlloDerm SELECT™ Regenerative Tissue
Matrix
 Meshed Rectangle
Meshed Rectangle
|
5 x 6 | 30 | X-Thin | 0.55 ± 0.25 | 141430M |
| Per request | Per request | X-Thin | 0.55 ± 0.25 | 141400M | |
AlloDerm SELECT™
Regenerative Tissue
Matrix
 Nonmeshed Rectangle
Nonmeshed Rectangle
| Product description | Piece size (cm) | Coverage (cm2) | Thickness | Thickness range (mm) | Product code |
|---|---|---|---|---|---|
AlloDerm SELECT™ Regenerative Tissue
Matrix
 Nonmeshed Rectangle
Nonmeshed Rectangle
|
Per request | Per request | X-Thin | 0.55 ± 0.25 | 141400 |
| Per request | Per request | Thin | 1.0 ± 0.2 | 141600 | |
ALLODERM SELECTTM REGENERATIVE TISSUE MATRIX
INDICATIONS AND IMPORTANT SAFETY INFORMATION
INDICATIONS
ALLODERM SELECTTM Regenerative Tissue Matrix (ALLODERM SELECTTM RTM refers to both ALLODERM SELECTTM RTM and ALLODERM SELECT RESTORETM RTM products) is intended to be used for repair or replacement of damaged or inadequate integumental tissue or for other homologous uses of human integument. This product is intended for single patient one-time use only. ALLODERM SELECTTM RTM is not indicated for use as a dural substitute or intended for use in veterinary applications.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ALLODERM SELECTTM RTM should not be used in patients with a known sensitivity to any of the antibiotics listed on the package and/or Polysorbate 20.
WARNINGS
Processing of the tissue, laboratory testing, and careful donor screening minimize the risk of the donor tissue transmitting disease to the recipient patient. As with any processed donor tissue, ALLODERM SELECTTM RTM is not guaranteed to be free of all pathogens. No long-term studies have been conducted to evaluate the carcinogenic or mutagenic potential or reproductive impact of the clinical application of ALLODERM SELECTTM RTM.
DO NOT re-sterilize ALLODERM SELECTTM RTM. DO NOT reuse once the tissue graft has been removed from the packaging and/or is in contact with a patient. Discard all open and unused portions of the product in accordance with standard medical practice and institutional protocols for disposal of human tissue. Once a package or container seal has been compromised, the tissue shall be either transplanted, if appropriate, or otherwise discarded. DO NOT use if the foil pouch is opened or damaged. DO NOT use if the seal is broken or compromised. DO NOT use if the temperature monitoring device does not display “OK.” DO NOT use after the expiration date noted on the label. Transfer ALLODERM SELECTTM RTM from the foil pouch aseptically. DO NOT place the foil pouch in the sterile field.
PRECAUTIONS
Poor general medical condition or any pathology that would limit the blood supply and compromise healing should be considered when selecting patients for implanting ALLODERM SELECTTM RTM as such conditions may compromise successful clinical outcome. Whenever clinical circumstances require implantation in a site that is contaminated or infected, appropriate local and/or systemic anti-infective measures should be taken.
ALLODERM SELECTTM RTM has a distinct basement membrane (upper) and dermal surface (lower). When applied as an implant, it is recommended that the dermal side be placed against the most vascular tissue. Soak the tissue for a minimum of 2 minutes using a sterile basin and room temperature sterile saline or room temperature sterile lactated Ringer’s solution to cover the tissue. If any hair is visible, remove using aseptic technique before implantation.
ALLODERM SELECTTM RTM should be hydrated and moist when the package is opened. DO NOT use if this product is dry. Use of this product is limited to specific health professionals (e.g., physicians, dentists, and/or podiatrists). Certain considerations should be made to reduce the risk of adverse events when performing surgical procedures using a tissue graft. Please see the Instructions for Use (IFU) for more information on patient/product selection and surgical procedures involving tissue implantation before using ALLODERM SELECTTM RTM.
ADVERSE EVENTS
The most commonly reported adverse events associated with the implant of a tissue graft include, but are not limited to the following: wound or systemic infection; seroma; dehiscence; hypersensitive, allergic or other immune response; and sloughing or failure of the graft.
ALLODERM SELECTTM RTM is available by prescription only.
For more information, please see the Instructions for Use (IFU) for ALLODERM SELECTTM RTM available at www.allergan.com/AlloDermIFU or call 1.800.678.1605.
To report an adverse reaction, please call Allergan at 1.800.433.8871.
ALLODERM SELECTTM Regenerative Tissue Matrix
Indications and Important Safety Information
INDICATIONS
ALLODERM SELECTTM Regenerative Tissue Matrix (ALLODERM SELECTTM RTM refers to both ALLODERM SELECTTM RTM and ALLODERM SELECT RESTORETM RTM products) is intended to be used for repair or replacement of damaged or inadequate integumental tissue or for other homologous uses of human integument. This product is intended for single patient one-time use only. ALLODERM SELECTTM RTM is not indicated for use as a dural substitute or intended for use in veterinary applications.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ALLODERM SELECTTM RTM should not be used in patients with a known sensitivity to any of the antibiotics listed on the package and/or Polysorbate 20.
WARNINGS
Processing of the tissue, laboratory testing, and careful donor screening minimize the risk of the donor tissue transmitting disease to the recipient patient. As with any processed donor tissue, ALLODERM SELECTTM RTM is not guaranteed to be free of all pathogens. No long-term studies have been conducted to evaluate the carcinogenic or mutagenic potential or reproductive impact of the clinical application of ALLODERM SELECTTM RTM.
DO NOT re-sterilize ALLODERM SELECTTM RTM. DO NOT reuse once the tissue graft has been removed from the packaging and/or is in contact with a patient. Discard all open and unused portions of the product in accordance with standard medical practice and institutional protocols for disposal of human tissue. Once a package or container seal has been compromised, the tissue shall be either transplanted, if appropriate, or otherwise discarded. DO NOT use if the foil pouch is opened or damaged. DO NOT use if the seal is broken or compromised. DO NOT use if the temperature monitoring device does not display “OK.” DO NOT use after the expiration date noted on the label. Transfer ALLODERM SELECTTM RTM from the foil pouch aseptically. DO NOT place the foil pouch in the sterile field.
PRECAUTIONS
Poor general medical condition or any pathology that would limit the blood supply and compromise healing should be considered when selecting patients for implanting ALLODERM SELECTTM RTM as such conditions may compromise successful clinical outcome. Whenever clinical circumstances require implantation in a site that is contaminated or infected, appropriate local and/or systemic anti-infective measures should be taken.
ALLODERM SELECTTM RTM has a distinct basement membrane (upper) and dermal surface (lower). When applied as an implant, it is recommended that the dermal side be placed against the most vascular tissue. Soak the tissue for a minimum of 2 minutes using a sterile basin and room temperature sterile saline or room temperature sterile lactated Ringer’s solution to cover the tissue. If any hair is visible, remove using aseptic technique before implantation.
ALLODERM SELECTTM RTM should be hydrated and moist when the package is opened. DO NOT use if this product is dry. Use of this product is limited to specific health professionals (e.g., physicians, dentists, and/or podiatrists). Certain considerations should be made to reduce the risk of adverse events when performing surgical procedures using a tissue graft. Please see the Instructions for Use (IFU) for more information on patient/product selection and surgical procedures involving tissue implantation before using ALLODERM SELECTTM RTM.
ADVERSE EVENTS
The most commonly reported adverse events associated with the implant of a tissue graft include, but are not limited to the following: wound or systemic infection; seroma; dehiscence; hypersensitive, allergic or other immune response; and sloughing or failure of the graft.
ALLODERM SELECTTM RTM is available by prescription only.
For more information, please see the Instructions for Use (IFU) for ALLODERM SELECTTM RTM.
To report an adverse reaction, please call Allergan at 1.800.433.8871.
See our new privacy terms at https://privacy.abbvie/.