CONTACT US
CONTACT US
CUSTOMER SERVICE
For ordering, billing, or finding your local
sales representative,
call 1.800.367.5737 or email
PRMOrder@allergan.com
ONLINE ORDERING
Click here: Allergan DirectCLINICAL AND MEDICAL ASSISTANCE
Call 1.800.678.1605 or visit our
Medical Information website
REIMBURSEMENT SUPPORT
Call 1.888.543.3656 or email
AllerganPRM@thepinnaclehealthgroup.com
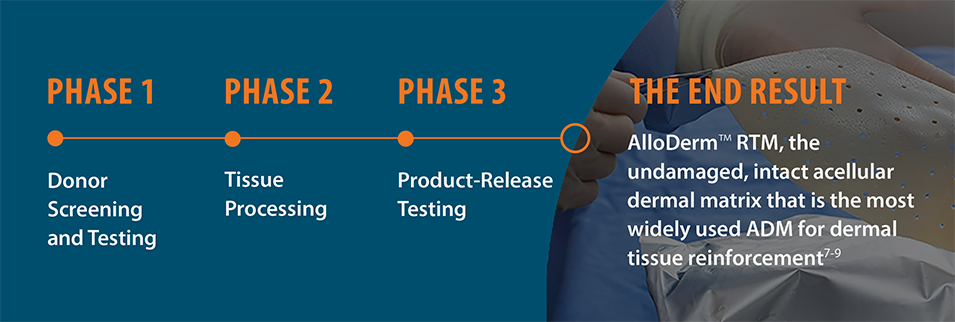
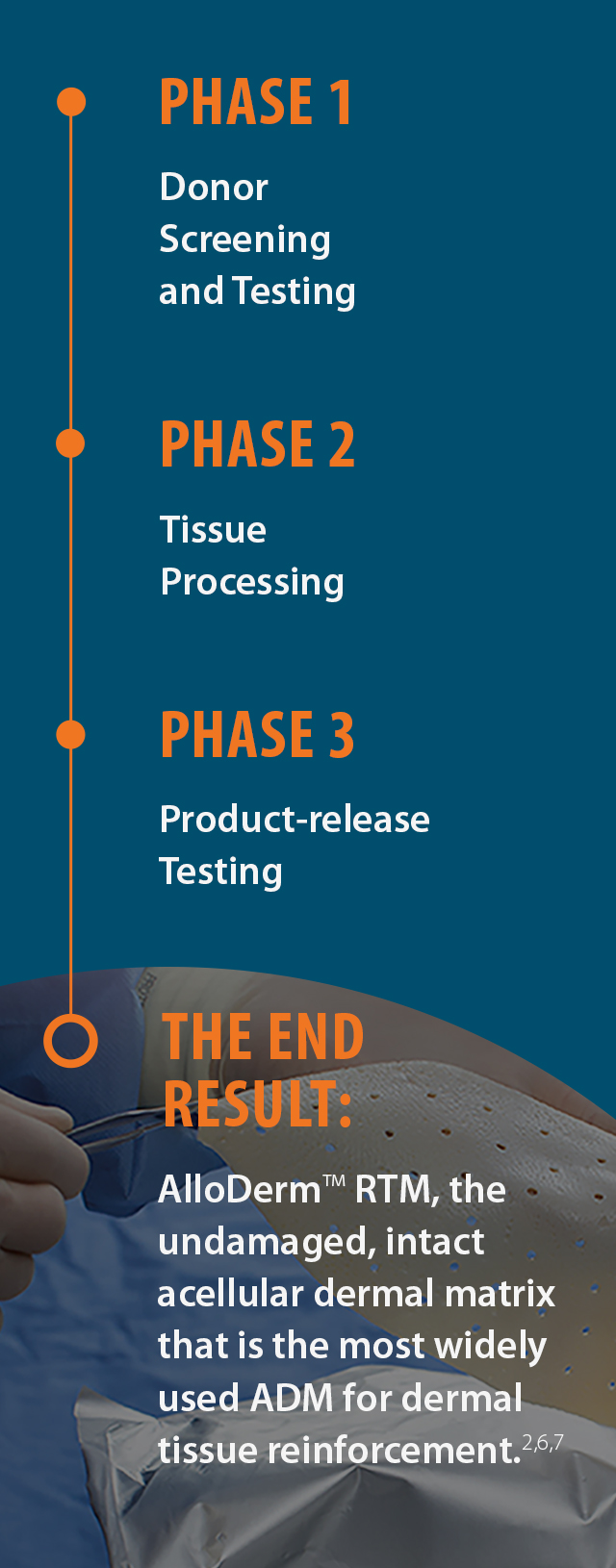
No other ADM has the
extensive experience
of AlloDermTM RTM



Most-used ADM
The industry leader, available since 1994.1,2*



Most-published ADM
Over 1,000 scientific† and clinical publications.3



4 million
implantations4



Free of
documented disease transmissions5


Proprietary LifeCell Tissue Process
Assures sterility while maintaining properties that support regeneration.6-8†
LEARN MORE





Extensive
portfolio
A range of products to meet the evolving needs of surgeons and their patients.
LEARN MORE


AlloDermTM RTM strikes
the balance between
sterility and regeneration
The LifeCell Tissue Process assures sterility
while maintaining matrix properties that
support regeneration.6-8





The only tissue processing facility to receive the exclusive ENERGY STAR Award in 2022.
Not all surgical scaffolds are the same: Regeneration is key
AlloDermTM RTM is an undamaged, intact acellular dermal matrix that enables positive recognition and supports regeneration, as demonstrated in preclinical models6-8†
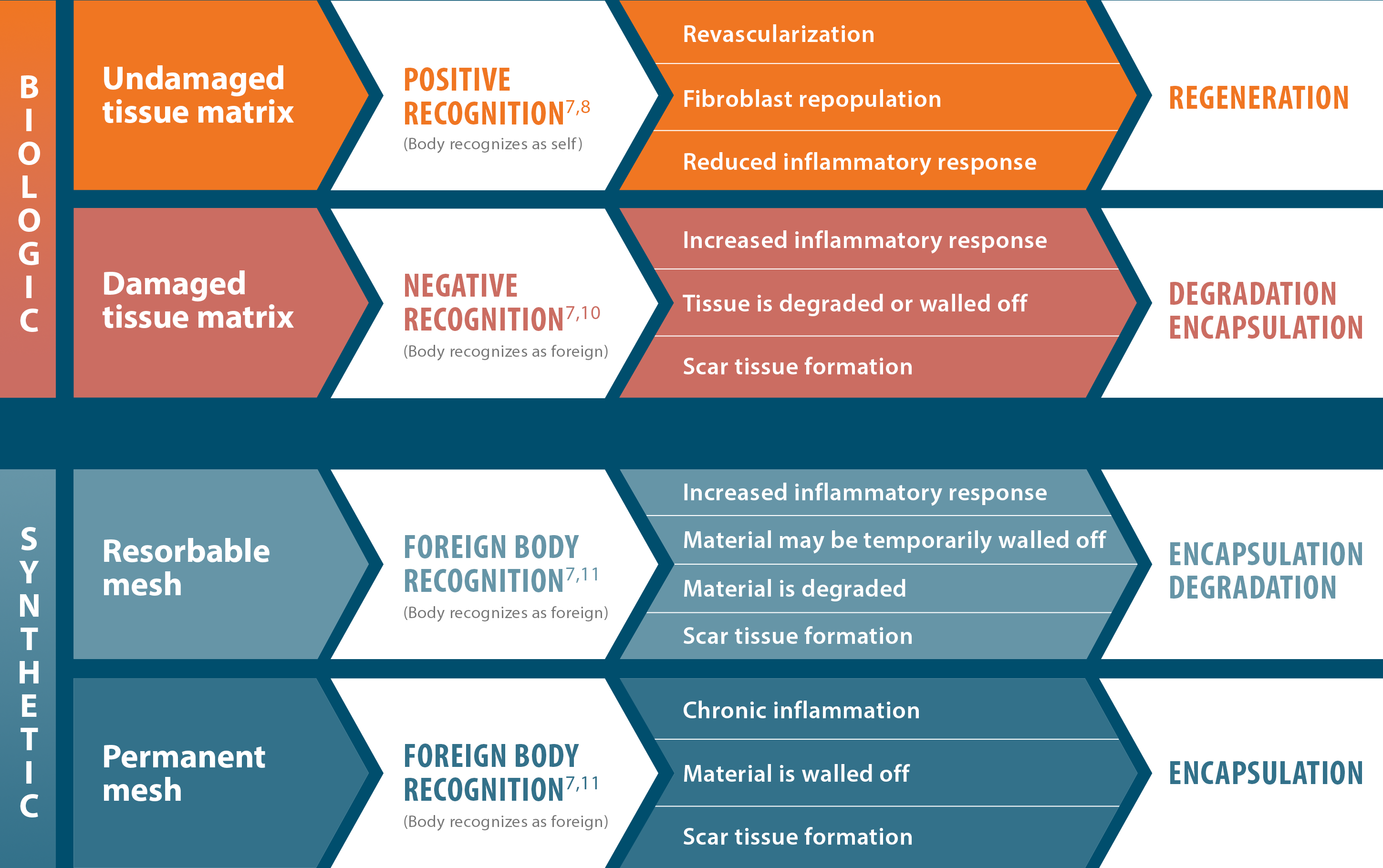


†Correlation of these results, based on animal studies, to results in humans has not been established.
An ongoing commitment
to innovation
Our extensive product portfolio aligns
with the evolving
clinical needs of
surgeons and their patients.



title
View product portfolio





AlloDerm™ RTM on YouTube
ALLODERM SELECTTM REGENERATIVE TISSUE MATRIX
INDICATIONS AND IMPORTANT SAFETY INFORMATION
INDICATIONS
ALLODERM SELECTTM Regenerative Tissue Matrix (ALLODERM SELECTTM RTM refers to both ALLODERM SELECTTM RTM and ALLODERM SELECT RESTORETM RTM products) is intended to be used for repair or replacement of damaged or inadequate integumental tissue or for other homologous uses of human integument. ALLODERM SELECTTM RTM is intended for use in post-mastectomy breast reconstruction surgical procedures where the use of the acellular dermal matrix (ADM) is considered homologous, such as managing a potential skin defect created from harvesting tissue for use in autologous tissue reconstruction. Examples of uses in post-mastectomy breast reconstruction not considered homologous include use of an ADM to form an extension of the submuscular pocket for placement of a breast implant or tissue expander, and use to prevent expander or implant extrusion, or to constrain the expander or implant in the correct position. This product is intended for use in one patient, on a single occasion. ALLODERM SELECTTM RTM is not indicated for use as a dural substitute or intended for use in veterinary applications.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ALLODERM SELECTTM RTM should not be used in patients with a known sensitivity to any of the antibiotics listed on the package and/or Polysorbate 20.
WARNINGS
Processing of the tissue, laboratory testing, and careful donor screening minimize the risk of the donor tissue transmitting disease to the recipient patient. As with any processed donor tissue, ALLODERM SELECTTM RTM is not guaranteed to be free of all pathogens. No long-term studies have been conducted to evaluate the carcinogenic or mutagenic potential or reproductive impact of the clinical application of ALLODERM SELECTTM RTM.
DO NOT re-sterilize ALLODERM SELECTTM RTM. DO NOT reuse once the tissue graft has been removed from the packaging and/or is in contact with a patient. Discard all open and unused portions of the product in accordance with standard medical practice and institutional protocols for disposal of human tissue. Once a package or container seal has been compromised, the tissue shall be either transplanted, if appropriate, or otherwise discarded. DO NOT use if the foil pouch is opened or damaged. DO NOT use if the seal is broken or compromised. DO NOT use if the temperature monitoring device does not display “OK”. DO NOT use after the expiration date noted on the label. Transfer ALLODERM SELECTTM RTM from the foil pouch aseptically. DO NOT place the foil pouch in the sterile field.
PRECAUTIONS
Poor general medical condition or any pathology that would limit the blood supply and compromise healing should be considered when selecting patients for implanting ALLODERM SELECTTM RTM as such conditions may compromise successful clinical outcome. Whenever clinical circumstances require implantation in a site that is contaminated or infected, appropriate local and/or systemic anti-infective measures should be taken.
ALLODERM SELECTTM RTM has a distinct basement membrane (upper) and dermal surface (lower). When applied as an implant, it is recommended that the dermal side be placed against the most vascular tissue. Soak the tissue for a minimum of 2 minutes using a sterile basin and room temperature sterile saline or room temperature sterile lactated Ringer’s solution to cover the tissue. If any hair is visible, remove using aseptic technique before implantation.
ALLODERM SELECTTM RTM should be hydrated and moist when the package is opened. DO NOT use if this product is dry. Use of this product is limited to specific health professionals (e.g., physicians, dentists, and/or podiatrists). Certain considerations should be made to reduce the risk of adverse events when performing surgical procedures using a tissue graft. Please see the Instructions for Use (IFU) for more information on patient/product selection and surgical procedures involving tissue implantation before using ALLODERM SELECTTM RTM.
ADVERSE EVENTS
Potential adverse events which may result from surgical procedures associated with the implant of a tissue graft include, but are not limited to, the following: wound or systemic infection; seroma; dehiscence; hypersensitive, allergic or other immune response; and sloughing or failure of the graft.
ALLODERM SELECTTM RTM is available by prescription only.
For more information, please see the Instructions for Use (IFU) for ALLODERM SELECTTM RTM.
To report an adverse reaction, please call Allergan Aesthetics at 1.800.433.8871.
ALLODERM SELECTTM Regenerative Tissue Matrix
Indications and Important Safety Information
INDICATIONS
ALLODERM SELECTTM Regenerative Tissue Matrix (ALLODERM SELECTTM RTM refers to both ALLODERM SELECTTM RTM and ALLODERM SELECT RESTORETM RTM products) is intended to be used for repair or replacement of damaged or inadequate integumental tissue or for other homologous uses of human integument. ALLODERM SELECTTM RTM is intended for use in post-mastectomy breast reconstruction surgical procedures where the use of the acellular dermal matrix (ADM) is considered homologous, such as managing a potential skin defect created from harvesting tissue for use in autologous tissue reconstruction. Examples of uses in post-mastectomy breast reconstruction not considered homologous include use of an ADM to form an extension of the submuscular pocket for placement of a breast implant or tissue expander, and use to prevent expander or implant extrusion, or to constrain the expander or implant in the correct position. This product is intended for use in one patient, on a single occasion. ALLODERM SELECTTM RTM is not indicated for use as a dural substitute or intended for use in veterinary applications.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ALLODERM SELECTTM RTM should not be used in patients with a known sensitivity to any of the antibiotics listed on the package and/or Polysorbate 20.
WARNINGS
Processing of the tissue, laboratory testing, and careful donor screening minimize the risk of the donor tissue transmitting disease to the recipient patient. As with any processed donor tissue, ALLODERM SELECTTM RTM is not guaranteed to be free of all pathogens. No long-term studies have been conducted to evaluate the carcinogenic or mutagenic potential or reproductive impact of the clinical application of ALLODERM SELECTTM RTM.
DO NOT re-sterilize ALLODERM SELECTTM RTM. DO NOT reuse once the tissue graft has been removed from the packaging and/or is in contact with a patient. Discard all open and unused portions of the product in accordance with standard medical practice and institutional protocols for disposal of human tissue. Once a package or container seal has been compromised, the tissue shall be either transplanted, if appropriate, or otherwise discarded. DO NOT use if the foil pouch is opened or damaged. DO NOT use if the seal is broken or compromised. DO NOT use if the temperature monitoring device does not display “OK”. DO NOT use after the expiration date noted on the label. Transfer ALLODERM SELECTTM RTM from the foil pouch aseptically. DO NOT place the foil pouch in the sterile field.
PRECAUTIONS
Poor general medical condition or any pathology that would limit the blood supply and compromise healing should be considered when selecting patients for implanting ALLODERM SELECTTM RTM as such conditions may compromise successful clinical outcome. Whenever clinical circumstances require implantation in a site that is contaminated or infected, appropriate local and/or systemic anti-infective measures should be taken.
ALLODERM SELECTTM RTM has a distinct basement membrane (upper) and dermal surface (lower). When applied as an implant, it is recommended that the dermal side be placed against the most vascular tissue. Soak the tissue for a minimum of 2 minutes using a sterile basin and room temperature sterile saline or room temperature sterile lactated Ringer’s solution to cover the tissue. If any hair is visible, remove using aseptic technique before implantation.
ALLODERM SELECTTM RTM should be hydrated and moist when the package is opened. DO NOT use if this product is dry. Use of this product is limited to specific health professionals (e.g., physicians, dentists, and/or podiatrists). Certain considerations should be made to reduce the risk of adverse events when performing surgical procedures using a tissue graft. Please see the Instructions for Use (IFU) for more information on patient/product selection and surgical procedures involving tissue implantation before using ALLODERM SELECTTM RTM.
ADVERSE EVENTS
Potential adverse events which may result from surgical procedures associated with the implant of a tissue graft include, but are not limited to, the following: wound or systemic infection; seroma; dehiscence; hypersensitive, allergic or other immune response; and sloughing or failure of the graft.
ALLODERM SELECTTM RTM is available by prescription only.
For more information, please see the Instructions for Use (IFU) for ALLODERM SELECTTM RTM.
To report an adverse reaction, please call Allergan Aesthetics at 1.800.433.8871.
See our new privacy terms at https://privacy.abbvie/.