CONTACT US
CONTACT US
CUSTOMER SERVICE
For ordering, billing, or finding your local
sales representative,
call 1.800.367.5737 or email
PRMOrder@allergan.com
ONLINE ORDERING
Click here: Allergan DirectCLINICAL AND MEDICAL ASSISTANCE
Call 1.800.678.1605 or visit our
Medical Information website
REIMBURSEMENT SUPPORT
Call 1.888.543.3656 or email
AllerganPRM@thepinnaclehealthgroup.com
Resources to support you
Choose a resource below to learn more:
AlloDermTM Guarantee Program
The AlloDermTM Guarantee Program offers facility customers a replacement of any piece of AlloDermTM RTM that is explanted, subject to the program terms and conditions.



To be eligible for the guarantee, facilities must comply with all terms and conditions. For more information, contact your local Allergan Aesthetics representative today, or call Allergan Aesthetics Customer Service at 1.800.367.5737.



Terms and Conditions
The AlloDermTM RTM Guarantee Program applies to all pieces of AlloDermTM RTM that are explanted, provided that the product has been used:- As intended by appropriately qualified and licensed surgeons
- In accordance with the current AlloDermTM RTM Instructions for Use, found at www.allergan.com/AlloDermIFU
Reimbursement for AlloDermTM RTM


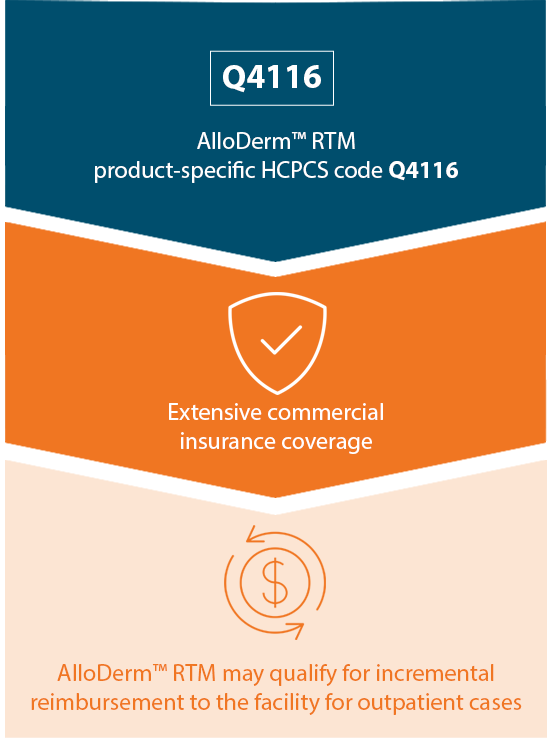
Reimbursement Hotline
The Reimbursement Hotline is our commitment to surgeons and facilities in their use of AlloDermTM RTM.
- Coding and payment
- Insurance coverage and documentation
- Case management for benefit verification and prior authorizations (PAs)
- Appeals support for denied PAs and/or claims
Contact us: AllerganPRM@thepinnaclehealthgroup.com
Providing physicians, hospitals, ambulatory surgical centers, and patients with comprehensive support
Reimbursement support includes:

Appeals assistance
Providing personalized appeals support for denied patient claims, including peer-to-peer reviews.

Coverage and
payment assistance
Conducting benefit verifications and confirming overall benefit structure for patient.

Education
Educating on correct coding, insurance coverage, policy guidelines, payment methodologies, required documentation, and payer regulations.

Prior authorization
Assisting with predetermination/
precertification process, including checking PA requirements and documentation needs, as well as appeals of denied PAs.
Processing support
Reviewing claims to assist with correct coding guidelines, billing options, and coverage in support of accurate claims processing, including support with irregular denials and inconsistent payment.
Regional Reimbursement Support Team
Our regional team members are available to provide tailored reimbursement support for your use of AlloDerm™ RTM.
Your reimbursement representative can assist your office with:
Economics/value proposition†
Coding (physician, facility)
Understanding coverage
Reimbursement
Health policy (commercial, government)
Healthcare reform
We are here to support you. Contact your regional reimbursement representative today
Medical Support Team
If you have any medically related product inquiries, please call us at 1.800.678.1605, option 2, or visit https://www.abbviemedinfo.com/ for help from our medical support team. Based on your request, it will be triaged by one of the following team members:
Medical Information
Our Medical Information department serves to communicate information about our products by providing relevant journal articles, product literature, labeling information, and other relevant documentation.
Medical Affairs
Talk LIVE with a medical and scientific professional who serves as a scientific information resource to provide scientific and clinical data relating to the use of Allergan Aesthetics products, with the goal of optimizing patient care and treatment outcomes.
Regulatory licensing documentation
The below are frequently requested documents pertaining to Allergan Aesthetics certifications, registrations, and licensing.
AATB Certificate FDA Establishment Registration and Listing for Human Cells, Tissues, and Cellular- and Tissue-Based Products (HCT/Ps)U.S. state licenses
California Tissue Bank LicenseDelaware Tissue Bank Permit
Florida Tissue Bank Certificate
Illinois Renewal for Tissue Bank
Maryland Tissue Bank Permit
New York License for Tissue Bank Operation
Oregon Procurement Organizations/Tissue Bank Registry
Canada
CTO Registration Certificate From Health CanadaRegenerative Medicine Return Goods Policy
Downloadable materials



Our downloadable Safety and Quality brochure provides important information on donor screening, proprietary processing, and quality testing measures, as well as the final tissue characteristics for each piece of AlloDermTM RTM.
AlloDermTM RTM Safety and Quality Brochure


Learn more about our program that offers facility customers a replacement of any piece of AlloDermTM RTM that is explanted, subject to terms and conditions.
AlloDermTM RTM Guarantee Brochure


From our robust LifeCell Tissue Process through our extensive line of shapes, sizes, and thicknesses, see what makes AlloDermTM the leading ADM.
AlloDermTM RTM Portfolio Brochure


A resource to help HCPs discuss with their patients why AlloDermTM is the leading ADM and the appropriate choice for their procedure.
AlloDermTM RTM Patient BrochureAlloDermTM RTM publications
Choose a topic below for a relevant selection of recent articles, or access a full bibliography of publications on PubMed.
Scientific articles
Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair
Xu H, Wan H, Sandor M, et al. Tissue Eng Part A. 2008;14(12):2009-2019.Extracellular wound matrices: a novel regenerative tissue matrix (RTM) technology for connective tissue reconstruction
Harper JR, McQuillan DJ. Wounds. 2007;19(6):163-168.Human monocyte activation by biologic and biodegradable meshes in vitro
Orenstein SB, Qiao Y, Kaur M, Klueh U, Kreutzer DL, Novitsky YW. Surg Endosc. 2010;24(4):805-811.†Correlation of these results, based on animal studies, to results in humans has not been established.
Donor site reinforcement
Use of regenerative human acellular tissue (AlloDerm) to reconstruct the abdominal wall following pedicle TRAM flap breast reconstruction surgery.
Glasberg SB, D'Amico RA. Plast Reconstr Surg. 2006;118(1):8-15.Head and neck procedures
For specific article inquiries, please visit the Medical Information website or call 1.800.678.1605.Human acellular dermal matrix grafts for rhinoplasty
Sherris DA, Oriel BS. Aesthet Surg J. 2011;31(suppl 7):95S-100S.AlloDerm for dorsal nasal irregularities
Jackson IT, Yavuzer R. Plast Reconstr Surg. 2001;107(2):553-558.Cleft palate fistula closure utilizing acellular dermal matrix
Emodi O, Ginini JG, van Aalst JA, et al. Plast Reconstr Surg Glob Open. 2018;6(3):e1682. doi:10.1097/GOX.0000000000001682 Weber PC, Lambert PR, Cunningham CD III, Richardson MS, Genao RB. Am J Otolaryngol. 2002;23(3):148-152.Wound procedures
Reyzelman A, Crews RT, Moore JC, et al. Int Wound J. 2009;6(3):196-208. Winters CL, Brigido SA, Linden BA, Simmons M, Hartman JF, Wright ML. Adv Skin Wound Care. 2008;21(8):375-381. Brigido SA, Boc SF, Lopez RC. Orthopedics. 2004;27(1 suppl):s145-s149.Use of an acellular regenerative tissue matrix over chronic wounds
Stacey DH. Eplasty. 2013;13:e61. Access a full bibliography of publications on PubMedHelpful links
Professional organizations are invaluable sources of information and tools to help you stay up-to-date on the latest advancements in the field of plastic and reconstructive surgery. Visit the links below to explore their offerings:
American Society of Plastic SurgeonsThe Aesthetic Society
American Academy of Otolaryngology—Head and Neck Surgery
American Association of Oral and Maxillofacial Surgeons
Important information related to tissue donation is available through these resources:
SkinDonation.comAmerican Association of Tissue Banks (AATB)
Association of Organ Procurement Organizations (AOPO)
Donate Life America
ALLODERM SELECTTM REGENERATIVE TISSUE MATRIX
INDICATIONS AND IMPORTANT SAFETY INFORMATION
INDICATIONS
ALLODERM SELECTTM Regenerative Tissue Matrix (ALLODERM SELECTTM RTM refers to both ALLODERM SELECTTM RTM and ALLODERM SELECT RESTORETM RTM products) is intended to be used for repair or replacement of damaged or inadequate integumental tissue or for other homologous uses of human integument. ALLODERM SELECTTM RTM is intended for use in post-mastectomy breast reconstruction surgical procedures where the use of the acellular dermal matrix (ADM) is considered homologous, such as managing a potential skin defect created from harvesting tissue for use in autologous tissue reconstruction. Examples of uses in post-mastectomy breast reconstruction not considered homologous include use of an ADM to form an extension of the submuscular pocket for placement of a breast implant or tissue expander, and use to prevent expander or implant extrusion, or to constrain the expander or implant in the correct position. This product is intended for use in one patient, on a single occasion. ALLODERM SELECTTM RTM is not indicated for use as a dural substitute or intended for use in veterinary applications.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ALLODERM SELECTTM RTM should not be used in patients with a known sensitivity to any of the antibiotics listed on the package and/or Polysorbate 20.
WARNINGS
Processing of the tissue, laboratory testing, and careful donor screening minimize the risk of the donor tissue transmitting disease to the recipient patient. As with any processed donor tissue, ALLODERM SELECTTM RTM is not guaranteed to be free of all pathogens. No long-term studies have been conducted to evaluate the carcinogenic or mutagenic potential or reproductive impact of the clinical application of ALLODERM SELECTTM RTM.
DO NOT re-sterilize ALLODERM SELECTTM RTM. DO NOT reuse once the tissue graft has been removed from the packaging and/or is in contact with a patient. Discard all open and unused portions of the product in accordance with standard medical practice and institutional protocols for disposal of human tissue. Once a package or container seal has been compromised, the tissue shall be either transplanted, if appropriate, or otherwise discarded. DO NOT use if the foil pouch is opened or damaged. DO NOT use if the seal is broken or compromised. DO NOT use if the temperature monitoring device does not display “OK”. DO NOT use after the expiration date noted on the label. Transfer ALLODERM SELECTTM RTM from the foil pouch aseptically. DO NOT place the foil pouch in the sterile field.
PRECAUTIONS
Poor general medical condition or any pathology that would limit the blood supply and compromise healing should be considered when selecting patients for implanting ALLODERM SELECTTM RTM as such conditions may compromise successful clinical outcome. Whenever clinical circumstances require implantation in a site that is contaminated or infected, appropriate local and/or systemic anti-infective measures should be taken.
ALLODERM SELECTTM RTM has a distinct basement membrane (upper) and dermal surface (lower). When applied as an implant, it is recommended that the dermal side be placed against the most vascular tissue. Soak the tissue for a minimum of 2 minutes using a sterile basin and room temperature sterile saline or room temperature sterile lactated Ringer’s solution to cover the tissue. If any hair is visible, remove using aseptic technique before implantation.
ALLODERM SELECTTM RTM should be hydrated and moist when the package is opened. DO NOT use if this product is dry. Use of this product is limited to specific health professionals (e.g., physicians, dentists, and/or podiatrists). Certain considerations should be made to reduce the risk of adverse events when performing surgical procedures using a tissue graft. Please see the Instructions for Use (IFU) for more information on patient/product selection and surgical procedures involving tissue implantation before using ALLODERM SELECTTM RTM.
ADVERSE EVENTS
Potential adverse events which may result from surgical procedures associated with the implant of a tissue graft include, but are not limited to, the following: wound or systemic infection; seroma; dehiscence; hypersensitive, allergic or other immune response; and sloughing or failure of the graft.
ALLODERM SELECTTM RTM is available by prescription only.
For more information, please see the Instructions for Use (IFU) for ALLODERM SELECTTM RTM.
To report an adverse reaction, please call Allergan Aesthetics at 1.800.433.8871.
ALLODERM SELECTTM Regenerative Tissue Matrix
Indications and Important Safety Information
INDICATIONS
ALLODERM SELECTTM Regenerative Tissue Matrix (ALLODERM SELECTTM RTM refers to both ALLODERM SELECTTM RTM and ALLODERM SELECT RESTORETM RTM products) is intended to be used for repair or replacement of damaged or inadequate integumental tissue or for other homologous uses of human integument. ALLODERM SELECTTM RTM is intended for use in post-mastectomy breast reconstruction surgical procedures where the use of the acellular dermal matrix (ADM) is considered homologous, such as managing a potential skin defect created from harvesting tissue for use in autologous tissue reconstruction. Examples of uses in post-mastectomy breast reconstruction not considered homologous include use of an ADM to form an extension of the submuscular pocket for placement of a breast implant or tissue expander, and use to prevent expander or implant extrusion, or to constrain the expander or implant in the correct position. This product is intended for use in one patient, on a single occasion. ALLODERM SELECTTM RTM is not indicated for use as a dural substitute or intended for use in veterinary applications.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ALLODERM SELECTTM RTM should not be used in patients with a known sensitivity to any of the antibiotics listed on the package and/or Polysorbate 20.
WARNINGS
Processing of the tissue, laboratory testing, and careful donor screening minimize the risk of the donor tissue transmitting disease to the recipient patient. As with any processed donor tissue, ALLODERM SELECTTM RTM is not guaranteed to be free of all pathogens. No long-term studies have been conducted to evaluate the carcinogenic or mutagenic potential or reproductive impact of the clinical application of ALLODERM SELECTTM RTM.
DO NOT re-sterilize ALLODERM SELECTTM RTM. DO NOT reuse once the tissue graft has been removed from the packaging and/or is in contact with a patient. Discard all open and unused portions of the product in accordance with standard medical practice and institutional protocols for disposal of human tissue. Once a package or container seal has been compromised, the tissue shall be either transplanted, if appropriate, or otherwise discarded. DO NOT use if the foil pouch is opened or damaged. DO NOT use if the seal is broken or compromised. DO NOT use if the temperature monitoring device does not display “OK”. DO NOT use after the expiration date noted on the label. Transfer ALLODERM SELECTTM RTM from the foil pouch aseptically. DO NOT place the foil pouch in the sterile field.
PRECAUTIONS
Poor general medical condition or any pathology that would limit the blood supply and compromise healing should be considered when selecting patients for implanting ALLODERM SELECTTM RTM as such conditions may compromise successful clinical outcome. Whenever clinical circumstances require implantation in a site that is contaminated or infected, appropriate local and/or systemic anti-infective measures should be taken.
ALLODERM SELECTTM RTM has a distinct basement membrane (upper) and dermal surface (lower). When applied as an implant, it is recommended that the dermal side be placed against the most vascular tissue. Soak the tissue for a minimum of 2 minutes using a sterile basin and room temperature sterile saline or room temperature sterile lactated Ringer’s solution to cover the tissue. If any hair is visible, remove using aseptic technique before implantation.
ALLODERM SELECTTM RTM should be hydrated and moist when the package is opened. DO NOT use if this product is dry. Use of this product is limited to specific health professionals (e.g., physicians, dentists, and/or podiatrists). Certain considerations should be made to reduce the risk of adverse events when performing surgical procedures using a tissue graft. Please see the Instructions for Use (IFU) for more information on patient/product selection and surgical procedures involving tissue implantation before using ALLODERM SELECTTM RTM.
ADVERSE EVENTS
Potential adverse events which may result from surgical procedures associated with the implant of a tissue graft include, but are not limited to, the following: wound or systemic infection; seroma; dehiscence; hypersensitive, allergic or other immune response; and sloughing or failure of the graft.
ALLODERM SELECTTM RTM is available by prescription only.
For more information, please see the Instructions for Use (IFU) for ALLODERM SELECTTM RTM.
To report an adverse reaction, please call Allergan Aesthetics at 1.800.433.8871.
See our new privacy terms at https://privacy.abbvie/.