CONTACT US
CONTACT US
CUSTOMER SERVICE
For ordering, billing, or finding your local
sales representative,
call 1.800.367.5737 or email
PRMOrder@allergan.com
ONLINE ORDERING
Click here: Allergan DirectCLINICAL AND MEDICAL ASSISTANCE
Call 1.800.678.1605 or visit our
Medical Information website
REIMBURSEMENT SUPPORT
Call 1.888.543.3656 or email
AllerganPRM@thepinnaclehealthgroup.com
Performance: The AlloDermTM difference
Take a closer look: Regeneration is key
Choose a topic below to read more:
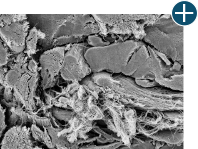
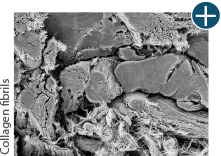
Tissue integrity
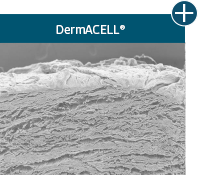
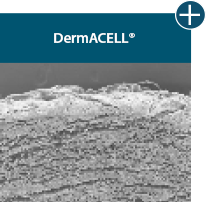
Not all processing is the same: AlloDermTM RTM processing maintains tissue integrity
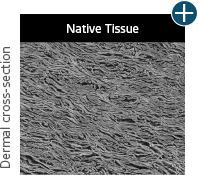
AlloDermTM RTM is minimally manipulated and gently processed to ensure it retains components critical to maintaining the biochemical and biomechanical integrity of the native tissue. As an undamaged tissue matrix, the native collagen architecture, including basement membrane structure, is maintained throughout the gentle LifeCell Tissue Process. An intact tissue matrix is vital for a positive immunologic response and regeneration.5-7*
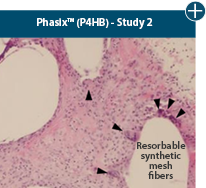
Dissimilar collagen structure
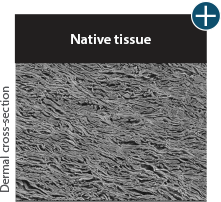

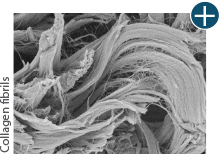


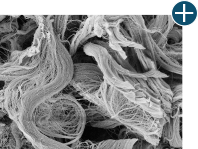
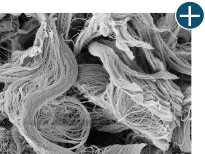
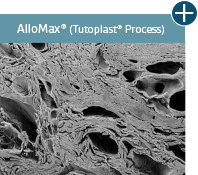
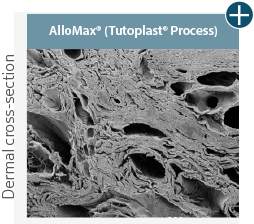
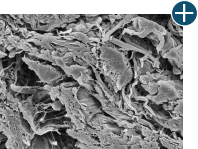
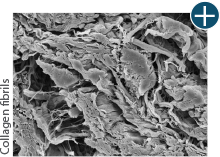
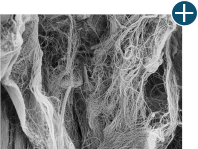
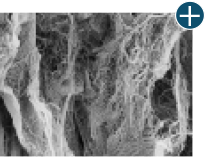
Body’s response
Maintaining tissue integrity is essential to achieving successful biological outcomes


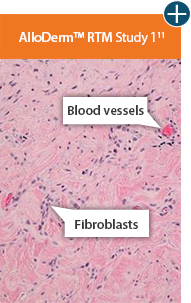
Cell repopulation and revascularization
AlloDermTM RTM supported rapid revascularization, fibroblast
repopulation, and remodeling in a preclinical model
An undamaged tissue matrix supports revascularization and cell repopulation. Damaged matrices experience delayed revascularization, which impedes white blood cell migration and fibroblast formation.6-8,10†
Take a closer look at AlloDermTM RTM
AlloDermTM RTM revealed widespread cellular infiltration with distribution of fibroblasts and numerous blood vessels.11 Damaged tissue matrices have a lack of fibroblast and blood vessel formation.12-14†
Widespread fibroblast &
blood vessel formation

The importance of cell repopulation and revascularization
Supports remodeling

Supports remodeling
Without vascular supply, there is no pathway for cells to remodel the tissue.15

Resists infection

Resists infection
Formation of intact vascular channels allows white blood cells to migrate to the site of an infection to minimize risk.16

Prevents necrosis

Prevents necrosis
Cellularized tissue matrices that do not revascularize will necrose.17
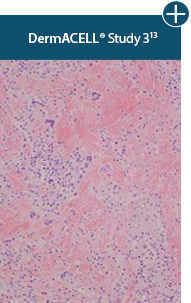
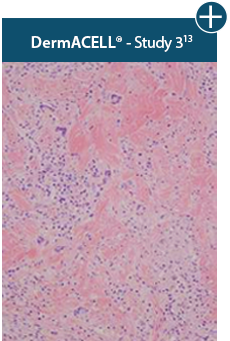
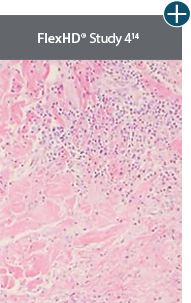
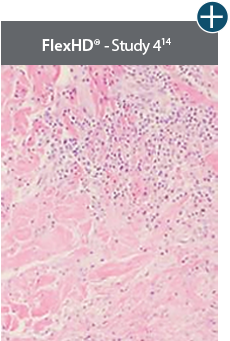
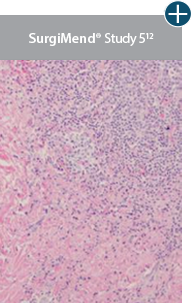
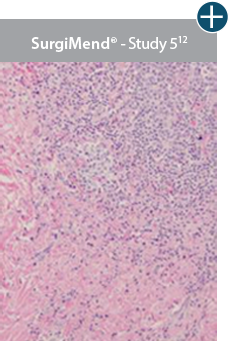
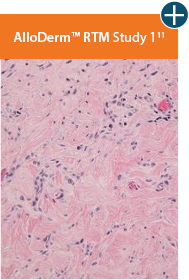
Minimal inflammation
AlloDermTM RTM demonstrated minimal inflammation in a preclinical model
Damaged matrices are viewed by the body as foreign and trigger a chronic inflammatory response that leads to matrix degradation or encapsulation, which may impede regeneration. An undamaged tissue matrix elicits a minimal inflammatory response because it retains the structure and function of the tissue to support regeneration.6-8†
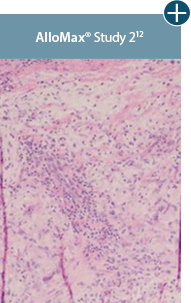
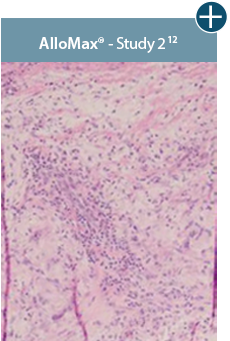
Take a closer look at AlloDermTM RTM
Through the preservation of an intact matrix, AlloDermTM RTM elicited a minimal
inflammatory response
and no evidence of
scar tissue formation in a preclinical model, as confirmed via the limited presence of macrophages and absence of
foreign-body giant cells. The
minimal inflammation contributes to the rapid and complete restoration of tissue during healing.
Damaged tissue matrices
elicit chronic inflammatory response demonstrated by foreign-body giant cells (noted by small
circles) and hypercellular
mixed inflammatory infiltrate (noted by dashed circle).11-14,18†
Minimal inflammatory
response
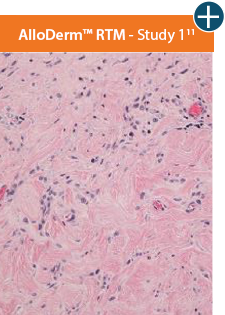
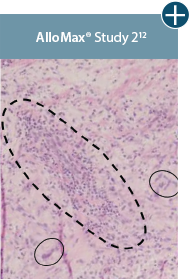
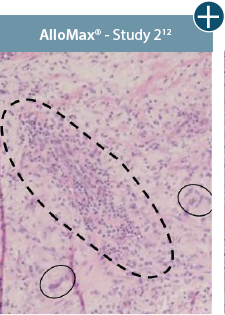
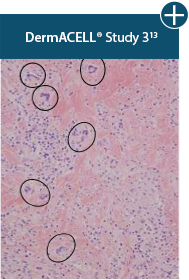
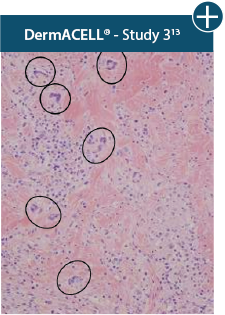
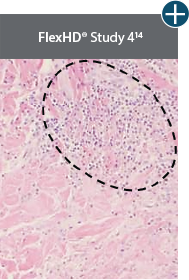
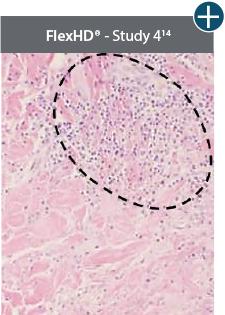
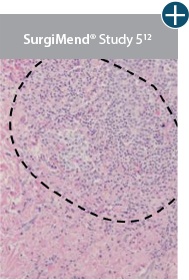
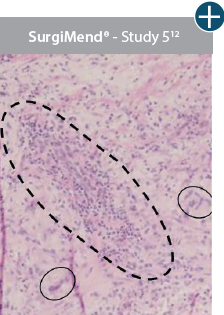
The negative effects of chronic inflammation

Prevents
remodeling

Prevents
remodeling
The body perceives a damaged tissue matrix as foreign, which may cause inflammation that impedes regeneration.6,7†

Provokes scar
formation

Provokes scar formation
Chronic inflammation often results in the formation of scar tissue. As inflammation increases, the rate of scar tissue formation is exacerbated.19,20†

Inhibits the ability
to fight infection

Inhibits the ability
to fight infection
Chronic inflammation may delay revascularization, which may impede fibroblast integration, blood vessel formation, and the ability to fight infection.21,22
Lack of scar formation
Positive recognition of AlloDermTM RTM supports a regenerative
rather than fibrotic response, as shown in preclinical models
The body will recognize materials differently and respond in one of two different ways: positive recognition or negative recognition. Excessive or prolonged inflammation from negative recognition often promotes fibrosis. The fibrotic process is the opposite of functional tissue regeneration. Positive outcomes are most often associated with minimized inflammatory response.7,11,20†
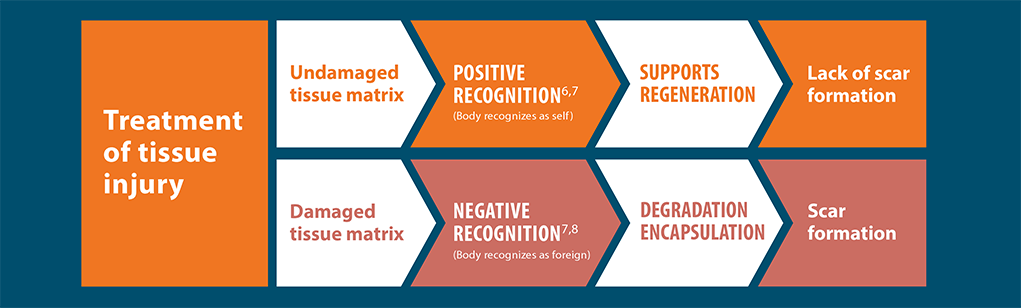

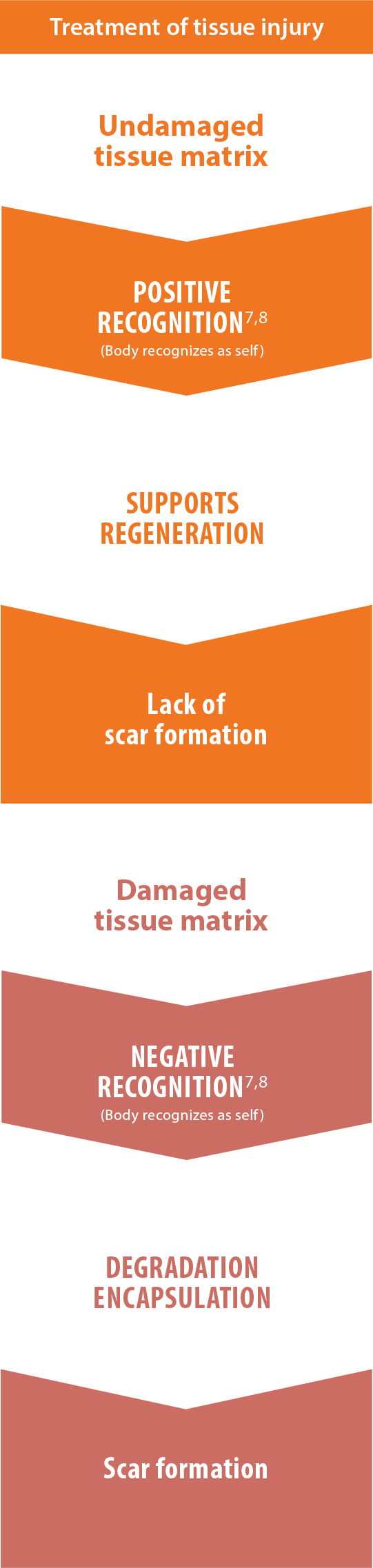
Wound healing cascade

Weak

Weak
Scar tissue has suboptimal functional, biomechanical, and physiological characteristics. It is weaker than normal fascia.19,20,23

Contractile

Contractile
A damaged tissue matrix is more likely to result in greater levels of scar and subsequent contracture. In a primate model, AlloDerm™ RTM demonstrated minimal contraction.8,11†

Causes visual deformities

Causes visual deformities
Graft contraction can cause a visual deformity (loss of domain) and reduce function and mobility of the tissue.19
Lack of resorption
AlloDermTM RTM did not demonstrate resorption of the graft in a primate model
Take a closer look at AlloDermTM RTM
An undamaged tissue matrix retains the structural and biomechanical properties that allow
for positive recognition,
supporting regeneration. Damaged matrices have altered structure and therefore are viewed by
the body as foreign, which
leads to contraction and resorption of the graft.5-8†
AlloDerm™ RTM showed minimal change in size 1 month after implantation in a primate model, demonstrating a lack
of resorption and minimal
contracture of surrounding tissue.11†
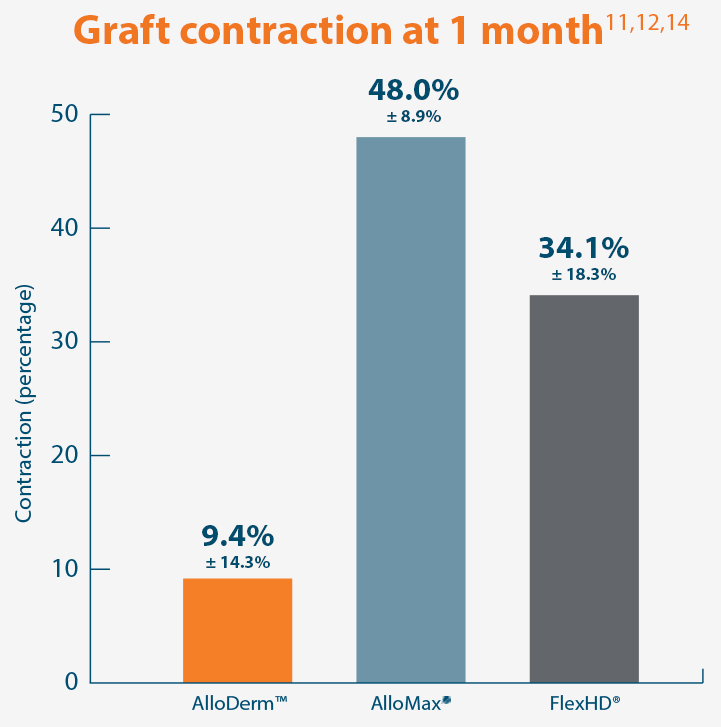


Baseline at time of placement
3 cm x 7 cm11,12,14
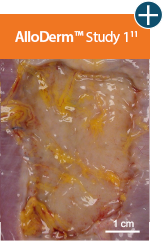
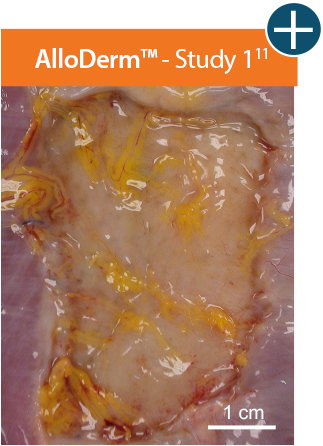
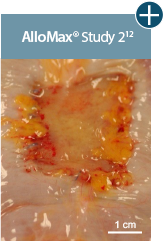
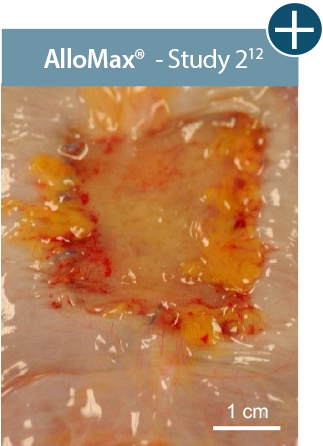
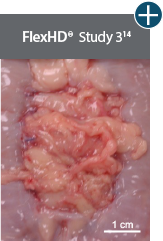
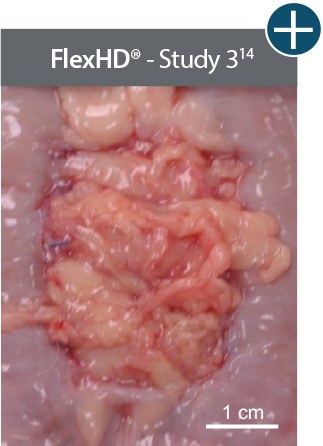
Representative gross photographs of tissue matrices evaluated following 1-month implantation in NHP-AWR model. Samples are taken from 3 different studies that followed the same protocol and were performed at the same institution at different times. All tissue matrices were fixated to the edges of a 3 x 7 cm full-thickness defect in the abdominal wall of nonhuman primates in an interpositional bridging configuration. †Correlation of these results, based on animal studies, to results in humans has not been established.
Consequences of resorption

Loss of strength

Loss of strength
Damaged ADMs demonstrated a loss of graft strength due to resorption.8,24

Loss of support

Loss of support
Damaged ADMs have demonstrated decreased biomechanical healing strength and diminished graft integrity.24

Replaced
with scar

Replaced with scar
A damaged tissue matrix may lead to degradation, resulting in partial or complete resorption and replacement of collagen matrix fibers with scar tissue.6,8,25
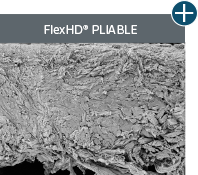
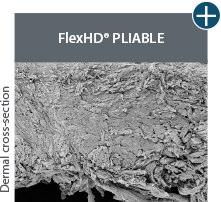
Pliability and handling
AlloDermTM RTM offers desired pliability and handling26
An undamaged tissue matrix retains elastin, which functions in close association with collagen to provide elasticity and shape retention. AlloDermTM RTM revealed widespread evidence of elastin in out-of-package staining.6,7,27
Take a closer look at AlloDermTM RTM
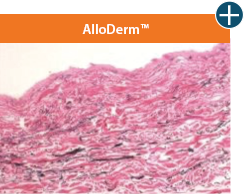
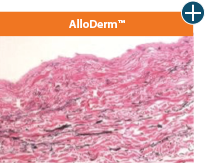
The maintenance of graft integrity in AlloDermTM RTM, including intact collagen fibers and elastin microfibrils, allows for surgeon-preferred handling and pliability. AlloDermTM RTM provides appropriate mechanical properties, including tensile strength and elasticity, both out-of-package as well as following implantation.5-7,26†
AlloDermTM RTM demonstrated pliability and drapeability out-of-package28*
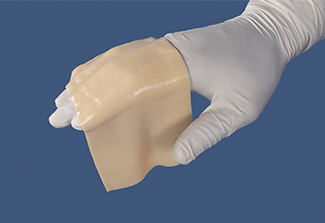


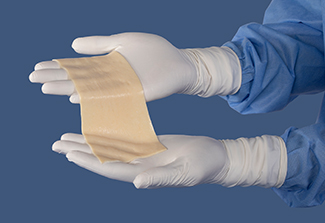


Importance of pliability*

Conforms to defect

Conforms to defect
An undamaged tissue matrix retains characteristics of native tissue and is pliable and able to conform to a defect.5,29

Tissue apposition

Tissue apposition
Tissue needs to conform to defect to have appropriate apposition to the vascular surface to revascularize.30

Better handling

Ideal handling
Tissue processed using certain damaging reagents can affect tissue characteristics and collagen structure.5,9
ALLODERM SELECTTM REGENERATIVE TISSUE MATRIX
INDICATIONS AND IMPORTANT SAFETY INFORMATION
INDICATIONS
ALLODERM SELECTTM Regenerative Tissue Matrix (ALLODERM SELECTTM RTM refers to both ALLODERM SELECTTM RTM and ALLODERM SELECT RESTORETM RTM products) is intended to be used for repair or replacement of damaged or inadequate integumental tissue or for other homologous uses of human integument. ALLODERM SELECTTM RTM is intended for use in post-mastectomy breast reconstruction surgical procedures where the use of the acellular dermal matrix (ADM) is considered homologous, such as managing a potential skin defect created from harvesting tissue for use in autologous tissue reconstruction. Examples of uses in post-mastectomy breast reconstruction not considered homologous include use of an ADM to form an extension of the submuscular pocket for placement of a breast implant or tissue expander, and use to prevent expander or implant extrusion, or to constrain the expander or implant in the correct position. This product is intended for use in one patient, on a single occasion. ALLODERM SELECTTM RTM is not indicated for use as a dural substitute or intended for use in veterinary applications.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ALLODERM SELECTTM RTM should not be used in patients with a known sensitivity to any of the antibiotics listed on the package and/or Polysorbate 20.
WARNINGS
Processing of the tissue, laboratory testing, and careful donor screening minimize the risk of the donor tissue transmitting disease to the recipient patient. As with any processed donor tissue, ALLODERM SELECTTM RTM is not guaranteed to be free of all pathogens. No long-term studies have been conducted to evaluate the carcinogenic or mutagenic potential or reproductive impact of the clinical application of ALLODERM SELECTTM RTM.
DO NOT re-sterilize ALLODERM SELECTTM RTM. DO NOT reuse once the tissue graft has been removed from the packaging and/or is in contact with a patient. Discard all open and unused portions of the product in accordance with standard medical practice and institutional protocols for disposal of human tissue. Once a package or container seal has been compromised, the tissue shall be either transplanted, if appropriate, or otherwise discarded. DO NOT use if the foil pouch is opened or damaged. DO NOT use if the seal is broken or compromised. DO NOT use if the temperature monitoring device does not display “OK”. DO NOT use after the expiration date noted on the label. Transfer ALLODERM SELECTTM RTM from the foil pouch aseptically. DO NOT place the foil pouch in the sterile field.
PRECAUTIONS
Poor general medical condition or any pathology that would limit the blood supply and compromise healing should be considered when selecting patients for implanting ALLODERM SELECTTM RTM as such conditions may compromise successful clinical outcome. Whenever clinical circumstances require implantation in a site that is contaminated or infected, appropriate local and/or systemic anti-infective measures should be taken.
ALLODERM SELECTTM RTM has a distinct basement membrane (upper) and dermal surface (lower). When applied as an implant, it is recommended that the dermal side be placed against the most vascular tissue. Soak the tissue for a minimum of 2 minutes using a sterile basin and room temperature sterile saline or room temperature sterile lactated Ringer’s solution to cover the tissue. If any hair is visible, remove using aseptic technique before implantation.
ALLODERM SELECTTM RTM should be hydrated and moist when the package is opened. DO NOT use if this product is dry. Use of this product is limited to specific health professionals (e.g., physicians, dentists, and/or podiatrists). Certain considerations should be made to reduce the risk of adverse events when performing surgical procedures using a tissue graft. Please see the Instructions for Use (IFU) for more information on patient/product selection and surgical procedures involving tissue implantation before using ALLODERM SELECTTM RTM.
ADVERSE EVENTS
Potential adverse events which may result from surgical procedures associated with the implant of a tissue graft include, but are not limited to, the following: wound or systemic infection; seroma; dehiscence; hypersensitive, allergic or other immune response; and sloughing or failure of the graft.
ALLODERM SELECTTM RTM is available by prescription only.
For more information, please see the Instructions for Use (IFU) for ALLODERM SELECTTM RTM.
To report an adverse reaction, please call Allergan Aesthetics at 1.800.433.8871.
ALLODERM SELECTTM Regenerative Tissue Matrix
Indications and Important Safety Information
INDICATIONS
ALLODERM SELECTTM Regenerative Tissue Matrix (ALLODERM SELECTTM RTM refers to both ALLODERM SELECTTM RTM and ALLODERM SELECT RESTORETM RTM products) is intended to be used for repair or replacement of damaged or inadequate integumental tissue or for other homologous uses of human integument. ALLODERM SELECTTM RTM is intended for use in post-mastectomy breast reconstruction surgical procedures where the use of the acellular dermal matrix (ADM) is considered homologous, such as managing a potential skin defect created from harvesting tissue for use in autologous tissue reconstruction. Examples of uses in post-mastectomy breast reconstruction not considered homologous include use of an ADM to form an extension of the submuscular pocket for placement of a breast implant or tissue expander, and use to prevent expander or implant extrusion, or to constrain the expander or implant in the correct position. This product is intended for use in one patient, on a single occasion. ALLODERM SELECTTM RTM is not indicated for use as a dural substitute or intended for use in veterinary applications.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
ALLODERM SELECTTM RTM should not be used in patients with a known sensitivity to any of the antibiotics listed on the package and/or Polysorbate 20.
WARNINGS
Processing of the tissue, laboratory testing, and careful donor screening minimize the risk of the donor tissue transmitting disease to the recipient patient. As with any processed donor tissue, ALLODERM SELECTTM RTM is not guaranteed to be free of all pathogens. No long-term studies have been conducted to evaluate the carcinogenic or mutagenic potential or reproductive impact of the clinical application of ALLODERM SELECTTM RTM.
DO NOT re-sterilize ALLODERM SELECTTM RTM. DO NOT reuse once the tissue graft has been removed from the packaging and/or is in contact with a patient. Discard all open and unused portions of the product in accordance with standard medical practice and institutional protocols for disposal of human tissue. Once a package or container seal has been compromised, the tissue shall be either transplanted, if appropriate, or otherwise discarded. DO NOT use if the foil pouch is opened or damaged. DO NOT use if the seal is broken or compromised. DO NOT use if the temperature monitoring device does not display “OK”. DO NOT use after the expiration date noted on the label. Transfer ALLODERM SELECTTM RTM from the foil pouch aseptically. DO NOT place the foil pouch in the sterile field.
PRECAUTIONS
Poor general medical condition or any pathology that would limit the blood supply and compromise healing should be considered when selecting patients for implanting ALLODERM SELECTTM RTM as such conditions may compromise successful clinical outcome. Whenever clinical circumstances require implantation in a site that is contaminated or infected, appropriate local and/or systemic anti-infective measures should be taken.
ALLODERM SELECTTM RTM has a distinct basement membrane (upper) and dermal surface (lower). When applied as an implant, it is recommended that the dermal side be placed against the most vascular tissue. Soak the tissue for a minimum of 2 minutes using a sterile basin and room temperature sterile saline or room temperature sterile lactated Ringer’s solution to cover the tissue. If any hair is visible, remove using aseptic technique before implantation.
ALLODERM SELECTTM RTM should be hydrated and moist when the package is opened. DO NOT use if this product is dry. Use of this product is limited to specific health professionals (e.g., physicians, dentists, and/or podiatrists). Certain considerations should be made to reduce the risk of adverse events when performing surgical procedures using a tissue graft. Please see the Instructions for Use (IFU) for more information on patient/product selection and surgical procedures involving tissue implantation before using ALLODERM SELECTTM RTM.
ADVERSE EVENTS
Potential adverse events which may result from surgical procedures associated with the implant of a tissue graft include, but are not limited to, the following: wound or systemic infection; seroma; dehiscence; hypersensitive, allergic or other immune response; and sloughing or failure of the graft.
ALLODERM SELECTTM RTM is available by prescription only.
For more information, please see the Instructions for Use (IFU) for ALLODERM SELECTTM RTM.
To report an adverse reaction, please call Allergan Aesthetics at 1.800.433.8871.
See our new privacy terms at https://privacy.abbvie/.